Case Studies
- Home
- Case Studies
- H2 Administration Reduces the Behavioral Deformities in the Offspring in a Maternal Immune Activation (MIA) Model

H2 Administration Reduces the Behavioral Deformities in the Offspring in a Maternal Immune Activation (MIA) Model
Introduction:
H2 administration, in several studies, has proven to be beneficial for the well-being of health. The study’s objective was to understand and investigate the long-term results of the offspring in the lipopolysaccharide (LPS)-induced maternal immune activation (MIA) model and the impact of maternal molecular hydrogen administration.
The researchers had earlier shown in the MIA mouse model the effects of the administration of H2. Hydrogen H2 administration reduced oxidative damage and neuroinflammation, pro-inflammatory cytokines, and microglial activation in the fetal brain.
Through tests like Y-maze, three-chamber, and pre-pulse inhibition (PPT) at postnatal 3 or 4 weeks, the researchers have evaluated the implications of H2 administration on short-term memory loss, sociability & social novelty, and sensorimotor gating. Oligodendrocytes and the number of neurons will also be analyzed at postnatal 5 weeks through immunohistochemical analysis. Maternal H2 administration considerably reduced LPS-induced abnormalities.
H2 Administration’s Effect on Behavioral Abnormalities of the Offspring – Background
- Feeling sad, wanting to cry, empty, desperate
- Excited or hot-tempered
- Feeling whatever you do is not good enough
- Increase the consumption of alcoholic beverages
- Indifferent to physical health and appearance
- The thought of death has an idea of how to end life
The association between maternal immune activation (MIA) and subsequent neurodevelopmental disorders in offspring has become increasingly recognized, with epidemiological evidence and research findings in various animal models. Maternal Immune Activation against viral or bacterial infections influences the developing fetal central nervous system (CNS), and increases the risk of schizophrenia and autism spectrum disorder (ASD) later in life. It has been suggested that MIA substantially impacts microglial development, and microglial disturbance disrupts neurogenesis, neuronal migration, and myelination, thus leading to consequent impairments in the offspring’s brain function. Based on these findings, maternal immune activationis believed to be a disease primer1. It has also been reported that MIA and MIA-induced fetal neuroinflammation stimulate the generation of reactive oxygen species (ROS) and disturbances in pro-inflammatory cytokine production in the fetal brain. These changes are reported to lead to direct and indirect death or dysfunction of neuronal and oligodendrocyte cells, which are considered the main targets of fetal brain injury.
We have previously suggested that the maternal administration of molecular hydrogen (H2) plays a neuroprotective role in the fetal brain against injury caused by oxidative stress and inflammation.
Ohsawa explained the effects of H2 as an antioxidant in their discovery. According to Ohsawa, “H2 is an antioxidant that selectively neutralizes hydroxyl radicals (•OH) and protects the brain from injury.”
Numerous successive studies have explored the therapeutic and preventive effects of H2 usage and indicated that H2 has other properties, including anti-inflammatory and anti-apoptotic effects. The safety and some futuristic benefits of H2 have already been proved and accepted in animal models and patients suffering from Parkinson’s disease, diabetes mellitus, and mild cognitive impairment.
However, no large randomized controlled trials have been conducted to date. Recently, researchers demonstrated that the administration of H2 to pregnant mouse dams significantly increased H2 concentration in the fetal brain, through the maternal-fetal interface, in a mouse model of lipopolysaccharide (LPS)-induced MIA. Fetal damage in this MIA model and the effect of maternal H2 administration on this damage can be summarized as follows:
Problems:
- MIA fetuses have a high mortality rate.
- Brain injury is associated with elevated levels of pro-inflammatory cytokines, aberrant microglial activation, and oxidative damage in the MIA fetal brain.
After administration of H2:
- Reduction of the above-mentioned adverse outcomes by maternal H2 administration.
This demonstrated the protective effect of maternal H2 administration on short-term outcomes in the MIA offspring. However, the long-term effects of the surviving offspring are yet to be investigated.
The current study used histological and behavioral examinations to investigate the long-term effects and efficacy of H2 administration in pregnant dams, as well as its neurological outcome in the MIA mouse model. Astrocytes, the most abundant cell population in the CNS, have a critical role in brain development. However, when atrocities are exposed to maternally-derived cytokines in Maternal Immune Activation, they produce various cytokines that lead to neurodevelopmental impairments. Furthermore, astrocyte activation is a known reason for the prolongation of brain damage and contributes to the formation of glial scars that limit neuronal plasticity. Thus, in the present study, Maternal Immune Activation and its modification by H2 administration were also investigated in vivo and in vitro.
Results
The researchers, in their article published in Scientific Reports, found the following results:
The results are quoted from the research paper to ensure the authenticity and precision of information communication.
Image Courtesy – Journal of Stroke and Cerebrovascular Diseases.
Maternal H2 administration restored offspring growth.
We investigated postnatal growth, which could be related to neurologic outcomes. From postnatal day (P) 6 to P10, offspring growth was significantly retarded in the LPS group compared to the control group (p < 0.05). Although significant differences were not observed between the LPS and LPS + HW groups, maternal administration of hydrogen water (HW) restored offspring growth to the control level.
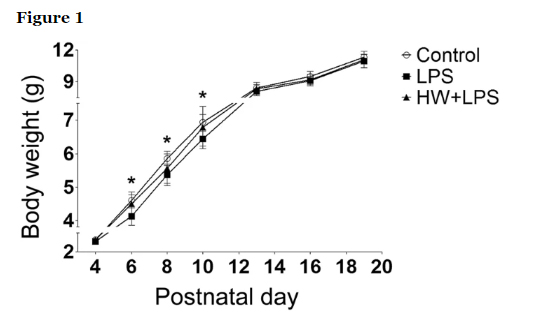
Offspring growth as evidenced by body weight (n = 17–22; Control, 17 pups/5 dams; LPS, 22 pups/5 dams; HW + LPS, 19 pups/5 dams); *p < 0.05, LPS group vs. Control group, by two-way repeated-measures ANOVA with Bonferroni post-hoc test. LPS, lipopolysaccharide; HW, hydrogen water.
Maternal H2 administration attenuated the behavioral deficits induced by LPS exposure.
Since MIA has been considered to cause behavioral abnormalities in offspring, including ASD/Schizophrenia-like behavior, we subsequently evaluated the effects of LPS and maternal administration of HW on short-term memory, sociability, social novelty, and sensorimotor gating. According to the figure below, spontaneous alternation was markedly reduced in the LPS group than in the control group (p < 0.001) in the Y-maze test, which indicates impaired short-term memory. Treatment with H2 significantly attenuated the LPS-induced short-term memory impairment (p < 0.05). There was no difference in the total number of arm entries among the three groups (Control, 24.0 ± 1.3; LPS, 22.6 ± 0.61; HW + LPS, 22.7 ± 0.91).
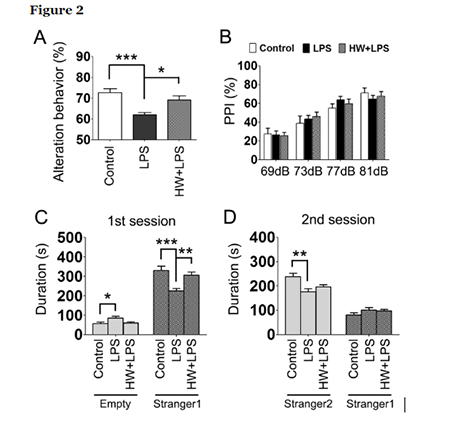
To investigate social behaviors, we evaluated sociability and preference for social novelty in the three-chambered social test. During the habituation phase, the mice in each group spent equal amounts of time exploring both compartments, with no biased preference for either empty cylinder. During the sociability phase, the mice in each group preferred spending more time in the chamber containing an unfamiliar mouse (stranger 1) relative to the opposite, empty chamber. However, the LPS-treated offspring approached the chamber containing stranger 1 significantly less frequently than the control and HW + LPS groups (p < 0.001 and p < 0.01, respectively; Fig. 2C). During the social novelty preference phase, the mice in each group showed a significant preference for the new, unfamiliar mouse (stranger 2) over the previous, now familiar mouse (stranger 1). However, the LPS-treated offspring approached the chamber containing the stranger 2 significantly less frequently than the control group (p < 0.01; Fig. 2D). There was no difference between the LPS and the HW + LPS groups, although a trend toward attenuation of the LPS-induced reduction in the preference for social novelty was detected in the HW + LPS group.
The pre-pulse inhibition (PPI) test was performed to evaluate sensorimotor integration by measuring the startle response to administered acoustic pulses; however, no significant differences were detected among the groups (Fig. 2B).
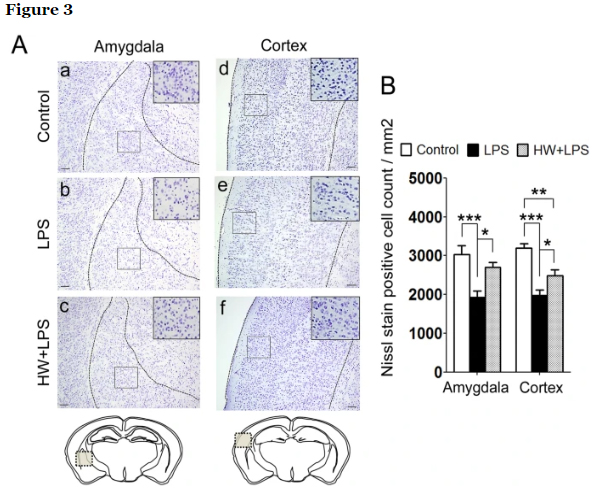
Maternal H2 administration attenuated the LPS-induced neuronal loss.
In our previous investigation, activated microglia were mostly accumulated in a temporal association area, 8 h after the LPS insult. Neurons in this area have been linked to sociability deficits. In addition, the association between autism and the amygdala, especially in the lateral amygdala, is well known. Moreover, the amygdala interacts with the hippocampus in relation to memory. Based on those findings, we subsequently performed Nissl staining and evaluated the number of neurons in the amygdala, cerebral cortex, and hippocampus to investigate the brain regions related to the behavioral deficits induced by LPS exposure. The quantitative results revealed significant reductions in the number of neurons in the amygdala and cerebral cortex in the LPS-treated offspring than in the control group (p < 0.001 and p < 0.001, respectively; Fig. 3A, panels a, b, d, e, and 3B), but not in the hippocampus. The LPS-induced neuronal loss in the amygdala and cerebral cortex was improved in the HW + LPS group than in the LPS group (p < 0.05 and p < 0.05, respectively; Fig. 3A, panels c, f and 3B).
Maternal H2 administration attenuated LPS-induced oligodendrocytic loss and suppressed astrocytic activation.
The pathophysiologic mechanisms of brain injury due to MIA are incompletely understood. However, during prenatal inflammation, activated astrocytes were reported to potentially harm neurons and oligodendrocytes, which would result in abnormal behaviors in later life. The number of oligodendrocytes was evaluated by using Olig2, a transcription factor involved in the differentiation of cells in the oligodendroglial lineage, and a mature oligodendrocyte marker. Compared with the control group, the number of Olig2-positive cells was clearly reduced in the white matter, amygdala, and cerebral cortex in the LPS group (p < 0.01, p < 0.05, and p < 0.05, respectively; Fig. 4A, panels a, b, d, e, g, h, and 4C). H2 treatment completely blocked the LPS-induced decrease of Olig2-positive cells in all three regions (p < 0.01, p < 0.05, and p < 0.01, respectively; Fig. 4A, panels c, f, i and 4C).
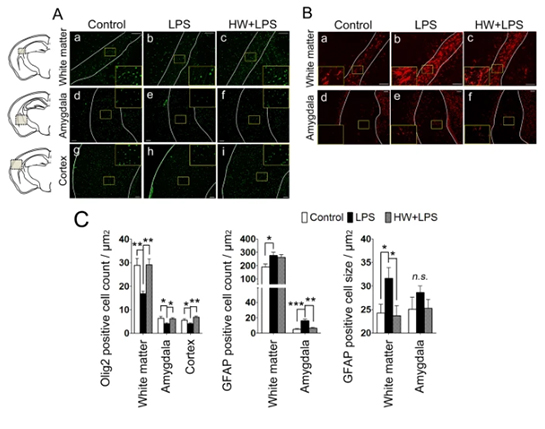
The hallmarks of astrocytic activation include the development of a hypertrophic morphology with fewer processes and upregulation of intermediate filament proteins, particularly glial fibrillary acidic protein (GFAP). In the control group, some GFAP-positive cells were detected, most of which were resting (Fig. 4B, panels a,d). Compared with the control group, a significantly increased number of activated astrocytes, characterized by a large soma and fewer processes (thus obtaining a more rounded shape), was observed in the white matter and amygdala of the LPS-treated offspring (p < 0.05 and p < 0.001, respectively; Fig. 4B, panel b, e and 4D). To evaluate the hypertrophic soma, the cellular size of the astrocytes was also quantified by calculating the ratio of GFAP immunostained area to the number of GFAP-positive cells. Larger soma was observed in the white matter of LPS-exposed mouse brains (p < 0.05; Fig. 4B, panels b and 4E). Although the number of GFAP-positive cells was unchanged following H2 treatment, the size of the GFAP-positive cells was significantly reduced in the white matter (p < 0.05; Fig. 4B, panels c and 4E), indicating a reduced number of reactive astrocytes by H2 treatment. In the amygdala, the number of GFAP-positive astrocytes in the HW + LPS group was significantly decreased (p < 0.01; Fig. 4B, panels f and 4D).
H2 treatment suppressed LPS-induced activation in primary cultured astrocytes.
Because the over-activation of immune cells, including astrocytes, could hurt neurons with the overproduction of pro-inflammatory cytokines, we investigated the anti-inflammatory effect of H2 on astrocytes using primary cultured astrocytes. As shown in Fig. 5, stimulation of astrocytes with LPS for three hours increased tumor necrosis factor α (TNF-α) (p < 0.001), interleukin (IL)-1β (p < 0.001), and IL-6 (p < 0.001) mRNA expression. These increases in expression of TNF-α (p < 0.01), IL-1β (p < 0.001) and IL-6 (p < 0.05) were significantly attenuated by H2 treatment.
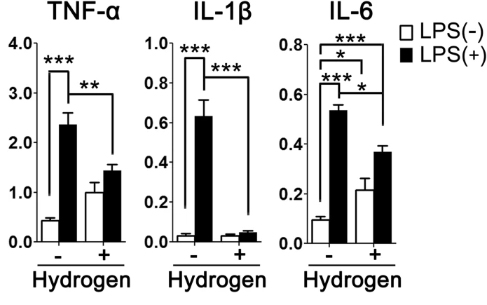
Altered gene expression patterns in primary cultured astrocytes from the control group, the LPS group, and the HW + LPS group.
As previously described, the gene expression profile of microglia and differential gene expression patterns of astrocytes from newborn mice in the control group, the LPS group, and the HW + LPS group were also observed. These results suggest that H2 might also influence the function of microglia and astrocytes via changes in gene expression.
Conclusion
In conclusion, it’s one more important discovery of the positive effects of H2 administration. This research has provided evidence that maternal H2 administration attenuates deficits in short-term memory and social interaction in the LPS-induced MIA model and has also demonstrated an attenuation of the LPS-induced neuronal and oligodendrocytic cell loss. The neuroprotective effect of H2 that appeared in the amygdala and cortex in this mouse model was remarkable. The neuroprotection was due to the repression of neuroinflammation, including excessive activation of microglia and astrocytes and the increased level of pro-inflammatory cytokines.
Though further investigation is required, the current results show the potential efficacy of maternal H2 administration for longer periods. It also yields long-term neurological results in the offspring exposed to inflammation in utero.
Overall, these findings indicate that maternal H2 administration exerts neuroprotective effects and ameliorates MIA-induced neurodevelopmental deficits of offspring later in life.
Humans have already started taking molecular H2 through methods. And, till date, there has not been a single reported case of any side effects of the molecular H2 administration.