Case Studies
- Home
- Case Studies
- Studying Molecular Hydrogen Gas Inhalation’s Effectiveness in the Treatment of Acute Cerebral Infarction

Studying Molecular Hydrogen Gas Inhalation's Effectiveness in the Treatment of Acute Cerebral Infarction
Introduction:
Humans have known about the benefits of molecular hydrogen since the late 18th century. Several studies have shown it to be well-tolerated, initially in animals and then in humans as well. Researchers have increasingly started researching to confirm and prove the beneficial effects of molecular hydrogen in mammalian physiology. Moreover, molecular hydrogen (H2) inhalation is increasingly becoming popular because of its therapeutic antioxidant benefits. However, most people are still a bit skeptical about the idea of utilizing this electrochemically neutral and non-polar diatomic compound.
Several studies prove the fact that the inhalation of molecular hydrogen (H2) is safe and can traverse the blood-brain barrier. One such study that evaluates the effects of inhalation of molecular hydrogen (H2) in humans with acute cerebral infarction was published in the Journal of Stroke and Cerebrovascular Diseases.
Below you can find the details of the study:
Background
- Feeling sad, wanting to cry, empty, desperate
- Excited or hot-tempered
- Feeling whatever you do is not good enough
- Increase the consumption of alcoholic beverages
- Indifferent to physical health and appearance
- The thought of death has an idea of how to end life
Molecular hydrogen (H2) behaves as a therapeutic antioxidant, and inhalation of H2 (1-4%) was markedly effective for treating cerebral infarction in a rat model. Furthermore, improvements were seen in a rat model of cardiac arrest when normoxic resuscitation was accompanied by molecular hydrogen inhalation. Also, the safety of H2 inhalation was conducted on subjects with post-cardiac arrest conditions and acute cerebral ischemia. Additionally, over 20 clinical experiences were conducted to examine the effectiveness of H2, including double-blinded pilot clinical studies. Therefore, a randomized and controlled clinical study is required to evaluate the overall positive effects of H2 inhalation in humans.
The study given below will show data from a randomized controlled clinical study that investigates the safety and efficaciousness of H2 treatment in patients suffering from acute cerebral infarction with National Institute of Health Stroke Scale Scores (NIHSS) 2–6. Extensive safety checks were conducted to examine the clinical conditions of patients. The aim of the study also included the motive to accelerate the evolvement of the H2 therapy into a novel therapy for real-life treatments.
Study Preparations and Choosing Participants
Researchers took 50 acute cerebral infarction patients for this randomized controlled study. The group of 50 was equally divided into two groups – the H2 group and the Control group. To randomize the selection, their ID numbers were used. Data collection and analysis were performed in a blinded manner as no nursing staff or any other staff or personnel knew the patients’ group names. The average age of patients was 73.3.0 in the Control group and 76.0 in the H2 group. Patients were enrolled between October 1, 2014, and January 28, 2016, and the final date of the study was February 28, 2016.
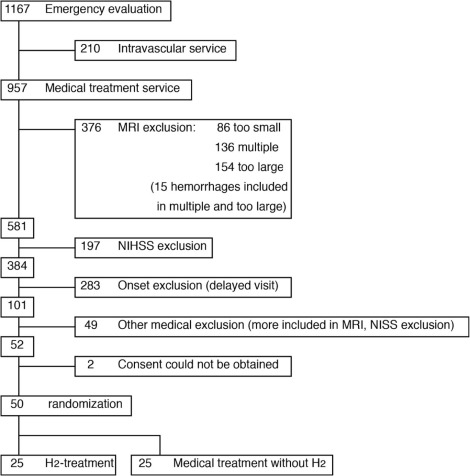
Image Courtesy – Journal of Stroke and Cerebrovascular Diseases.
Extensive Evaluation
To avoid and detect any abnormality, vital signs such as blood pressure, body temperature, food intake, O2 saturation, and body temperature were checked regularly three times every day. NIHSS scores were blindly recorded every day for 2 weeks. Moreover, the scores of the Barthel Index (BI), Brunnstrom Stage (BRS), Modified Rankin Scale (mRS), and Functional Independence Measure (FIM) were also registered in a blinded manner.
MRI scans were conducted on the 3rd, 5th, 7th, 10th, and 14th day from the day when the study began. The infarction site was named as the hyperintensity area in the diffusion-weighted image.
Quoting the study in the Journal of Stroke and Cerebrovascular Diseases:
“The abnormality was evaluated with the size (volume) and severity (MRI signal intensity). The size (=A) was obtained by manually surrounding the infarct core using the region of interest (ROI) software of the Digital Imaging and Communication in Medicine (DICOM) (Rosslyn, VA, USA) and by automatic counting. The severity (B and C) was obtained as the average of the MRI signal intensity of pixels in the infarct core ROI (=B) and the contralateral normal brain ROI (=C) of precisely the same size and mirror-image location, also automatically by the DICOM software. The signal intensity ratio between the infarct and the normal brain was calculated as B/C.
Diffusion-weighted images in the serial MRI scans were compared using the relative MRI signal intensity (=RSI).
The RSI used in this study was a product (A × B/C) of the size of the infarct (A) and the signal intensity ratio (B/C).
The calculation was repeated for all of the slices where the infarction extended with continuity. In the case of multiple infarcts, the calculation was obtained in the exact same fashion for all of the infarct sites. The data obtained were reproducible and reliable, as has been reported previously.”
Blood Test
Blood samples were withdrawn on days 1, 7, and 14 for regular tests to monitor blood counts and kidney, liver, pancreas, electrolytes, and cardiac enzymes. All the tests were conducted in a blinded manner. Moreover, on each patient’s admission, an electrocardiogram was ordered and was later done as and when needed.
Results
MRI Data – Lower RSI in H2 Group
No signs of intracerebral hemorrhage were observed in the infarction-hypersensitive area or other brain areas in the MRI study in both the H2 group and Control group. The relative MRI signal intensity (RSI) value on day 1 fluctuated between 28-855 with 241 as an average value for the H2 group and from 18 to 1895 with 272 as an average value (High RSI is directly proportional to the severity of the disease). However, subsequent changes in RSIs were observed between H2 and the Control group on the 7th and 14th day with P = .025 and .028, respectively (The p-value is a metric to judge results. If the p-value is equal to or less than 0.05, the result is deemed as significant, but if the p-value is higher than .05, the results are non-significant).
Furthermore, log transformation changed the total sequential data between the two groups. It was found significant with P = .002. This gave indications of improvements in the H2 group.
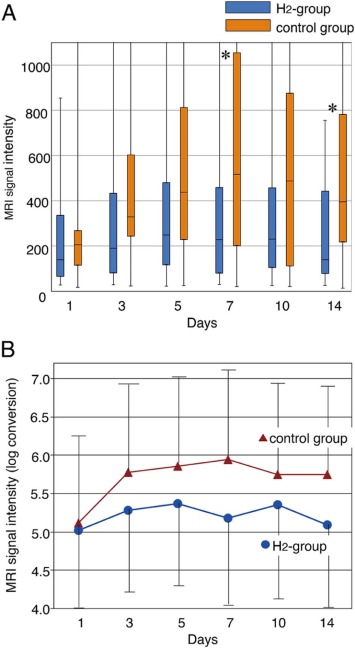
Image Courtesy – Journal of Stroke and Cerebrovascular Diseases.
If you refer to the graph given above, the later hike on the 10th day in the H2 group was minimal, somewhere around 152% of the day 1 data. The RSI on day 14 decreased and attained almost the normal range, with a fall of around 121% on day 1. If you compare this with the RSI of the Control group patients on the 14th day, the H2 group‘s RSI was on the lower side, and the Control group‘s RSI was still higher with 219% of the RSI on day 1 (Control group). This shows that the severity of pathological changes at the hypersensitive area (the infarction site) was less and was quickly near-normalized in the H2 group. It’s a positive sign as far as H2 inhalation’s safety and benefits are concerned.
Neurological Data - Improvement
National Institutes of Health Stroke Scale (NIHSS), which represents the neurological stats, displayed positive advancements in both groups. However, the improvements were more evident in the H2 group. Between the 3rd and 5th day from the start of the admission, the NIHSS score slightly increased in the Control group, with symptoms worsening. Nonetheless, there was no significant increase in the score in the H2 group.
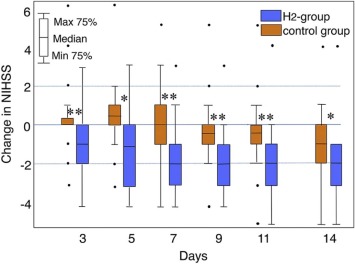
Image Courtesy – Journal of Stroke and Cerebrovascular Diseases.
After the 5th day, the scores started to indicate the improvement in the condition of the patients in both the groups, with a major difference between the two groups. In the H2 group, the severity of the disease decreased and was more apparent than in the control group. An excellent indication was that all the days, post-5th day, were statistically recorded as significant with P<.01 for days 5 and 14 and P <.001 for days 3, 7, 9 and 11.
Physical Therapy Improvement
The activities of daily living (ADL) capability of the patients was evaluated by physical therapy data using the BI, BRS, mRS, and FIM for the first 2 weeks. Because 9 patients (3 in the H2 group and 6 in the control group) could not go to the physical therapy department every day, the data from these patients was excluded from the final assessment.
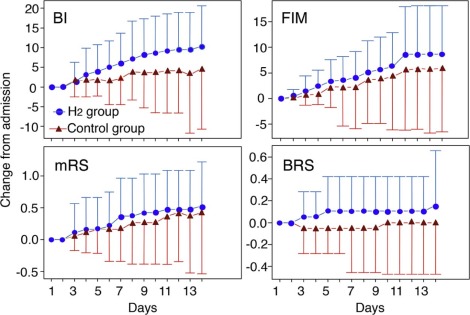
Image Courtesy – Journal of Stroke and Cerebrovascular Diseases.
Conclusion:
The inhalation of H2 gas was confirmed to be safe and efficacious in patients with acute cerebral infarction. Therefore, H2 gas therapy has the potential for actual application in acute cerebral infarction as a novel and safe therapeutic treatment.