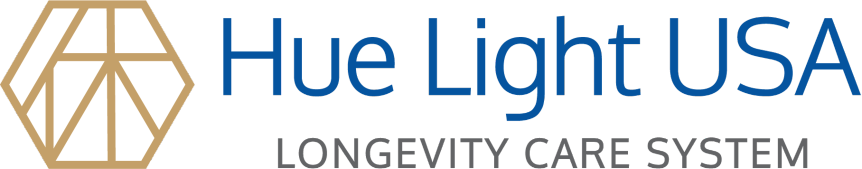Oxidative Stress Reduction and Increased BDNF Expression in the Hippocampus Through Photobiomodulation (660 nm) Therapy
Introduction:
Photobiomodulation therapy keeps people intrigued with its wide range of health benefits and usefulness in treating many diseases. Numerous studies by researchers and papers published show the positive effect of this miraculous therapy. Here the study will demonstrate the impact of PBM (660 nm) on the improvement of BDNF expression and reduction in oxidative stress.
Brain-derived neurotrophic factor (BDNF) affects particular types of neurons in the peripheral and central nervous system to accelerate the growth, survival, and categorization of new neurons and synapses.
BDNF is known to have a variety of effects on the nervous system. In the brain, it is expressed in the hippocampus, cortex, and basal brain and is involved in learning and memory. BDNF is essential for long-term memory and helps stimulate and regulate nerve development. During stress exposure, corticosterone appears to reduce the expression of BDNF in rats, and sustained stress causes atrophy in the hippocampus. It has been shown that atrophy in the hippocampus and other limbic systems occurs in people with depression. In a rat model of induced post-traumatic stress disorder, treatment with the antidepressant fluoxetine was shown to increase BDNF expression and exercise capacity.
Moreover, increased expression of BDNF in a genetic model of cognitive deficits leads to improved social and cognitive function. In addition, the expression of BDNF is reduced in an amyloid-β1-42 (Aβ1-42)-induced Alzheimer’s rat model, while BDNF administration was shown to increase cognitive function. As a result, BDNF acts on the nervous system and has excellent effects in the treatment of related diseases.
Photobiomodulation Therapy (PBMT)
Photobiomodulation therapy (PBMT), a treatment method using infrared or near-infrared light (600–1100 nm), is used in traumatic and degenerative brain diseases. PBMT (808 nm) irradiation could help cerebrospinal fluid (CSF) to induce neuronal differentiation of human umbilical cord mesenchymal stem cells (hUC-MSCs) in the early stage and can enhance mesenchymal stem cell (MSC) tissue repair, differentiation, and proliferation. Photoreceptor regulation of cytochrome c oxidase activity is the most important mechanism of action, and PBMT can enhance secondary metabolism by modulating mitochondrial function, neurotransmission, and redox status. This therapy is based on activating signaling pathways by photons in response to specific molecules in living organisms. Recently, PBMT has been clinically applied for a wide range of medical indications, including wound healing promotion; reduction of pain, edema, and inflammation caused by cell and tissue death; and differentiation and proliferation effects.
The purpose of this study was to see if photobiomodulation therapy at 660 nm reduces hippocampal cell damage.
Results
The researchers, Jin-Chul Heo, Ji-Ae Park, Dae-Kwang Kim, and Jong-Ha Lee, in their article published in Scientific Reports, found the following results regarding PBM therapy; the results are quoted from the research paper to ensure the authenticity and precision of information communication.
LED at 660 nm inhibits cell death by reducing oxidative stress
The hippocampal HT-22 cell line was used to assess the effects of H2O2-induced oxidative stress on cell survival and the cell death-suppressing effect of the 660 nm LED (Fig. 1A). After treating HT-22 cells with 100, 300, and 1000 μM H2O2, the cell viability was 72.7, 57.3, and 20.7%, respectively. In contrast, treatment with the 660 nm LED increased the cell viability to 88.4, 66.2, and 22.0%, respectively. The percent increase in cell viability in the presence of H2O2 at 100, 300, and 1000 µM was 15.7, 8.9, and 1.3%, respectively (Fig. 1B). The results show that the 660 nm LED suppresses the oxidative stress caused by H2O2, which increases the survival rate of cells exposed to oxidative stress.
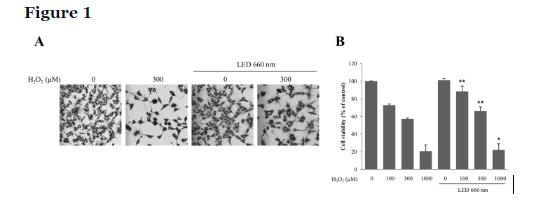
Image Courtesy – https://www.nature.com/articles/s41598-019-46490-4
LED at 660 nm increases BDNF expression in hippocampal cells through the activation of ERK and CREB signaling pathways
RT-PCR assessed the expression of BDNF in HT-22 cells. Irradiation of HT-22 cells with the 660 nm LED increased BDNF expression by about 2-fold. Although H2O2 treatment reduced BDNF expression, LED irradiation in the presence of H2O2 could still increase BDNF expression by approximately 2.1-fold. Melatonin at 1 mM was used as a positive control (Fig. 2A, B).
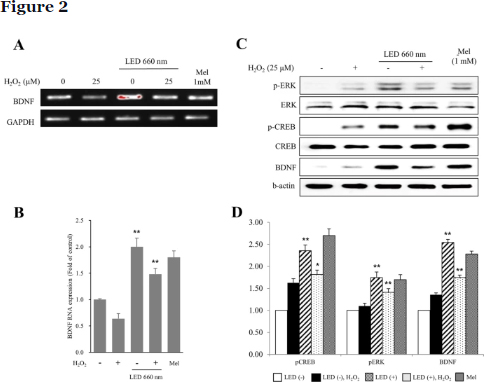
Image Courtesy – https://www.nature.com/articles/s41598-019-46490-4
To investigate the signal transduction pathway leading to BDNF upregulation, we examined ERK and CREB activation by assessing their phosphorylation levels. We found that phosphorylation of ERK and CREB was increased upon irradiation with the 660 nm LED light. p-ERK and p-CREB levels were increased by H2O2 treatment, further increasing upon LED irradiation at 660 nm (Fig. 2C, D). These results suggest that the ERK and CREB signal transduction pathways might mediate the increased BDNF expression in HT-22 cells following 660 nm LED irradiation.
LED at 660 nm increases the expression of BDNF in the mouse hippocampus
BDNF upregulation by LED irradiation was confirmed in the mouse hippocampus by immunohistochemistry. The number of BDNF-expressing cells was 2.5 and 2.8 times higher in the cases of LED treatment and H2O2 treatment, respectively, of mouse hippocampal organotypic slice cultures (Fig. 3A, B). We also discovered that the number of p-ERK- and p-CREB-positive cells was higher with LED irradiation than without. Upon LED irradiation, p-ERK-positive cells were increased by about 2- and 3.8-fold without or with H2O2, respectively. Under the same conditions, p-CREB was increased by about 1.6- and 3.3-fold, respectively. These results support the hypothesis that the increased levels of BDNF by LED in the hippocampus might be mediated by ERK and CREB signaling pathways.
Image Courtesy – https://www.nature.com/articles/s41598-019-46490-4
LED at 660 nm promotes the activity of antioxidant enzymes in the hippocampus
We examined the mRNA expression of BDNF and the antioxidant enzymes glutathione peroxidase (GPx), superoxide dismutase 1 (SOD1), and glutathione reductase (GR) in mouse hippocampal organotypic slice cultures irradiated with LED at 660 nm (Fig. 4). BDNF expression was increased in both control and H2O2-treated hippocampal slices upon LED irradiation. GPx expression was increased by approximately 2.7-fold by LED irradiation. However, expression was decreased after H2O2-induced oxidative stress. LED irradiation also increased SOD1 and GR levels by about 3- and 2.4-fold in control slices and SOD1 levels by approximately 1.6-fold in the H2O2-treated slices; in the latter condition, LED did not affect GR levels. These results show that LED at 660 nm induces BDNF expression in the hippocampus, as well as the expression of the antioxidant enzymes GPx, SOD1, and GR. In addition, the 660 nm LED increased the expression of SOD1 upon induction of oxidative stress by H2O2.
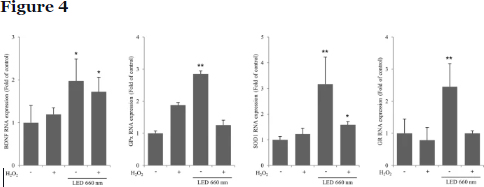
Image Courtesy – https://www.nature.com/articles/s41598-019-46490-4
LED at 660 nm enhances the activity of the antioxidant enzymes glutathione peroxidase (GPx), superoxide dismutase (SOD1), and glutathione reductase (GR). mRNA levels of antioxidant enzymes in organotypic hippocampal slices were examined by RT-qPCR after induction of oxidative stress by H2O2 (100 μM) and LED irradiation. Values represent the fold-change in expression levels compared to control. GPx, SOD1, and GR expressions increased in accordance with the increase in BDNF expression. NT is not treated. *p < 0.05 and **p < 0.01 vs. control.
Conclusion
The study has successfully demonstrated that 660 nm photobiomodulation therapy inhibited excessive apoptosis escalated by oxidative stress in hippocampal cell lines and boosted BDNF expression. Due to the consistent exposure of PBM light on wheat and soybean seeds with LED (638-731 nm), the total phenol content and the anti-oxidative properties of α-tocopherol and vitamin C increased significantly.
PBMT on ischemia-reperfusion injury in the abdominal muscle in a rat model has shown an increase in the activity of antioxidants.
The development of new treatments, especially drugs that utilize natural compounds, has been challenging due to cost and safety issues. Whereas, low-level light therapy, or Photobiomodulation, has been relatively safe and used by many around the world for an extended period. It is believed that the application of Photobiomodulation therapy will increase in the days to come and will help the human race treat various diseases.